Research Highlights:
The effect of ammonolysis conditions on the structural properties and oxidation kinetics of cubic niobium oxynitride
Graça, V.C.D.; Holz, L.I.V.; Loureiro, F.J.A.; Mather, G.C.; Fagg, D.P.
Niobium oxynitrides, especially, have been suggested for a diverse range of applications, including potential electrocatalysts (e.g., water splitting, nitrogen reduction, etc.), antibacterial agents, superconducting materials, and coatings, among others. In this work, we carefully explore the impact of ammonolysis conditions on the crystalline phase formation of niobium oxynitride compounds. Depending on the ammonolysis temperature and time, different composition-dependent changes in crystallographic structure can be induced across d-NbNxOy (cubic), Nb4(N,O)5 (tetragonal), NbN (hexagonal) phases. Potential defect-chemistry models were proposed to support measured compositional variations. A detailed kinetic analysis was then performed on the cubic materials to understand how composition can influence the thermal oxidation behaviour within this structure-type.
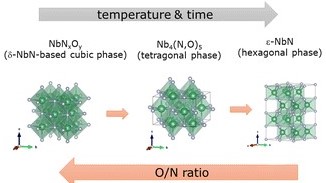
. Journal of Materials Chemistry C 11 29 (2023): 9960-9973, DOI 10.1039/d3tc01068f
Exploring the mixed transport properties of sulfur(VI)-doped Ba2In2O5 for intermediate-temperature electrochemical applications
D.Pérez-Coll, J.C.Pérez-Flores, N.Nasani, P.R.Slater,D.P.Fagg
The mixed (electronic–protonic–oxide-ionic) conducting properties of the stabilised orthorhombic perovskite Ba2In1.8S0.2O5+d were investigated in detail Appreciable mixed contributions to the electrical transport from protons, oxide ions and holes are confirmed between 550 and 700 °C
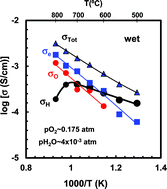
Journal of Materials Chemistry A, 4(2016)11069 - 11076
Enhancing electrochemical performance by control of transport properties in buffer layers-solid oxide fuel/electrolyser cells
D.Ramasamy, N.Nasani, A.D.Brandão, D.Pérez Coll, D.P.Fagg
Tailoring the transport properties of thin ceria-based buffer layers in solid oxide fuel or electrolyser cells can provide the necessary phase stability against chemical interaction at the electrolyte/electrode interface, while also providing radical improvements in the electrochemical performance of the oxygen electrode. This improvement can be related to increased levels of ambipolar conductivity in the mixed conducting buffer layer, which provides an additional parallel path for electrochemical reaction. This is an important breakthrough as it shows how electrode polarization resistance can be substantially improved, in otherwise identical electrochemical cells, solely by tailoring the transport properties of thin intermediate buffer layers.
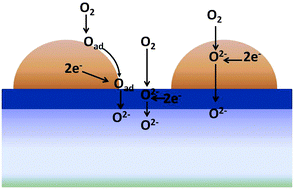
Physical Chemistry Chemical Physics, 17(17)(2015)11527-11539
Fabrication and electrochemical performance of a stable, anode supported thin BaCe0.4Zr0.4Y0.2O3-d electrolyte Protonic Ceramic Fuel Cell
N.Nasani, D.Ramasamy, S.Mikhalev, A.V.Kovalevsky, D.P.Fagg
Fabrication and electrochemical characterisation of a potential protonic ceramic fuel cell based on a Ni-BaZr0.85Y0.15O3-d anode supported thin film proton conducting BaCe0.4Zr0.4Y0.2O3-d electrolyte with a Pr2NiO4+d cathode. A thin (~5 µm), dense and crack free BaCe0.4Zr0.4Y0.2O3-d electrolyte film was successfully obtained on a porous anode support. Such a thin electrolyte was shown to not dominate the area specific resistance at low temperatures
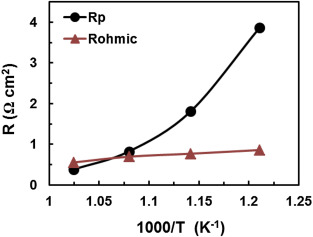
J.Power Sources, 278(2015)582-589
Synthesis of catalytically active rock salt structured MgxNb1-xO nanoparticles for MgH2 system.
D.Pukazhselvan, I.Antunes, S.Lo Russo, J. Perez, D.P.Fagg
XRD examination of 30 h ball milled MgH2 + Nb2O5 confirms the formation of a rock salt product MgxNb1-xO. It is shown that MgH2 catalyzed with the pre-made 2 wt.% MgxNb1-xO desorbs hydrogen at least 50 °C lower than the in-situ 2 wt.% Nb2O5 catalyzed MgH2 with improved reversible absorption. This result highlights that the addition of the MgxNb1-xO catalyst in a pre-reduced state can offer distinct performance advantages over its in-situ preparation.
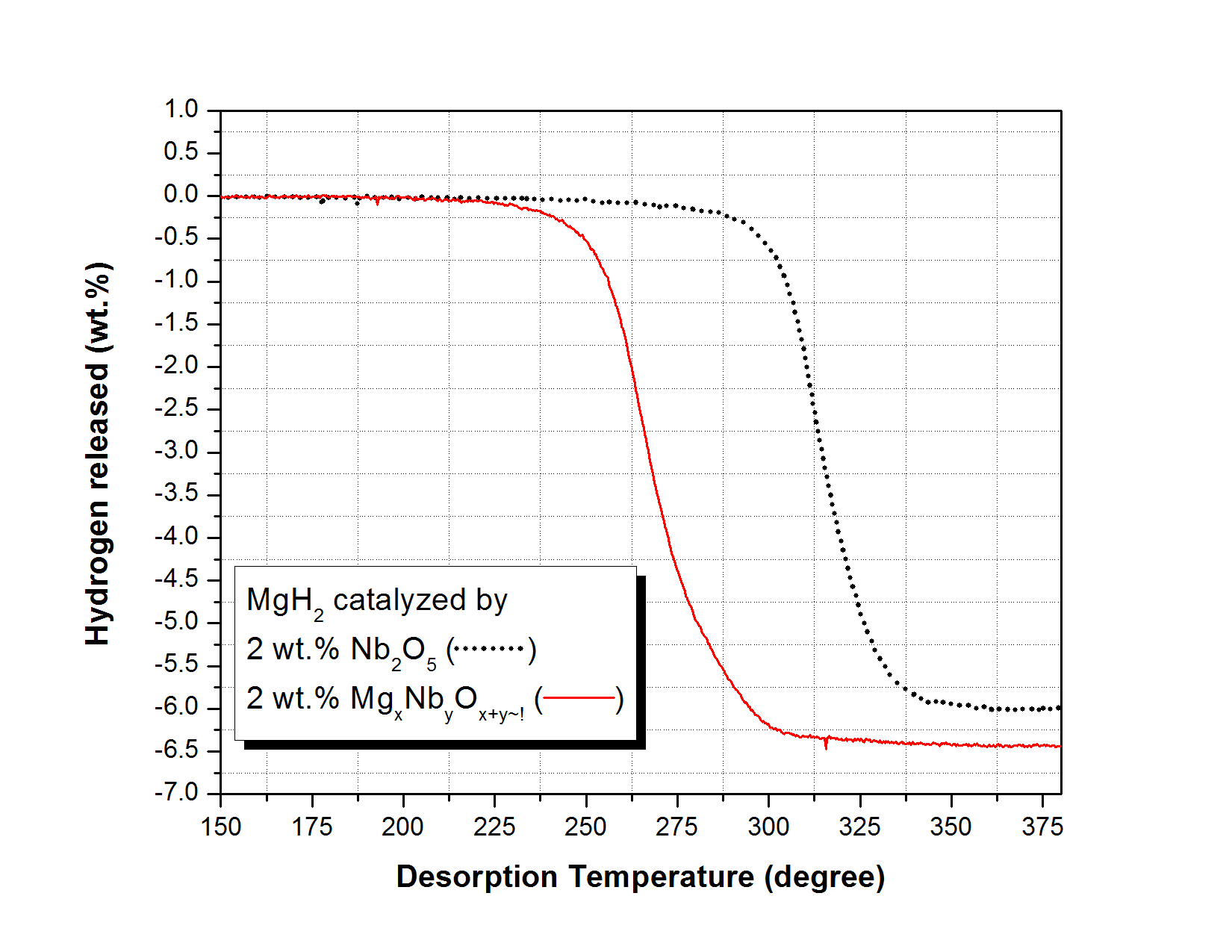
Int.J.Hydrogen Energy, 39(33)(2014)18984-18988
Enhanced BaZrO3 mechanosynthesis by the use of metastable ZrO2 precursors.
Z. Sherafat, I. Antunes, C. Almeida, J. R. Frade, M. H. Paydar, G. C. Mather and D. P. Fagg
This work assessed the impact of structural differences between stable and metastable ZrO2 precursors on the mechanochemical preparation of BaZrO3. Monoclinic (m-ZrO2) and tetragonal (t-ZrO2) zirconia polymorphs were prepared without stabilizing additives by slow alkaline precipitation.The t-ZrO2 precursor was shown to exhibit the higher reactivity with barium peroxide, yielding significantly earlier formation of barium zirconate under room-temperature mechanosynthesis.
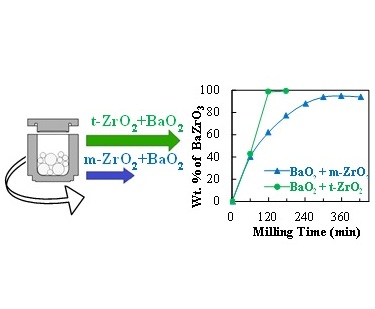
Dalton transactions, 43(2014)9324
Synthesis and conductivity of Ba(Ce,Zr,Y)O3-d electrolytes for PCFCs by new nitrate-free combustion method.
N.Narendar, P.A.N.Dias, J.Saraiva, D.P.Fagg
The new acetate–H2O2 combustion method was extended to synthesize BaCe0.8-xZrxY0.2O3-d (x = 0, 0.1, 0.4, 0.6 and 0.8) electrolyte membranes for proton ceramic fuel cells (PCFCs). The new acetate combustion method offers a cost effective, simple and environmentally friendly preparation route, which avoids the emission of NOx gases that are typical of traditional nitrate precursor routes. The route should also be applicable for the synthesis of other multi-element ceramic-oxide materials.
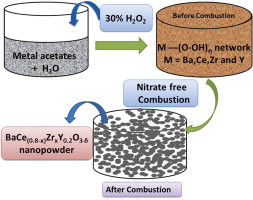
Int.J.Hydrogen Energy, 38 (20)(2013)8461-8470
The importance of phase purity in Ni-BaZr0.85Y0.15O3-d cermet anodes – Novel nitrate-free combustion route and electrochemical study.
N.Narendar, G.C.Mather, P.A.N.Dias, D.P.Fagg
A novel, nitrate-free, combustion method has been developed to prepare Ni-BaZr0.85Y0.15O3-d (Ni-BZY) cermet anodes for proton ceramic fuel cells. Nickel acetate and 30% H2O2 were used as starting precursors for the combustion reaction into which pre-prepared BZY powders were dispersed. The advantages of this nitrate-free, combustion method have been demonstrated by comparison to a more common nitrate/glycine combustion route. The results demonstrate that use of the nitrate-free precursors is essential to avoid partial decomposition of the pre-prepared BZY phase. Partial decomposition of the perovskite phase limits performance by increasing the higher frequency polarisation resistance and this phenomenon is suggested to be associated with impaired proton transport in the oxide cermet phase.
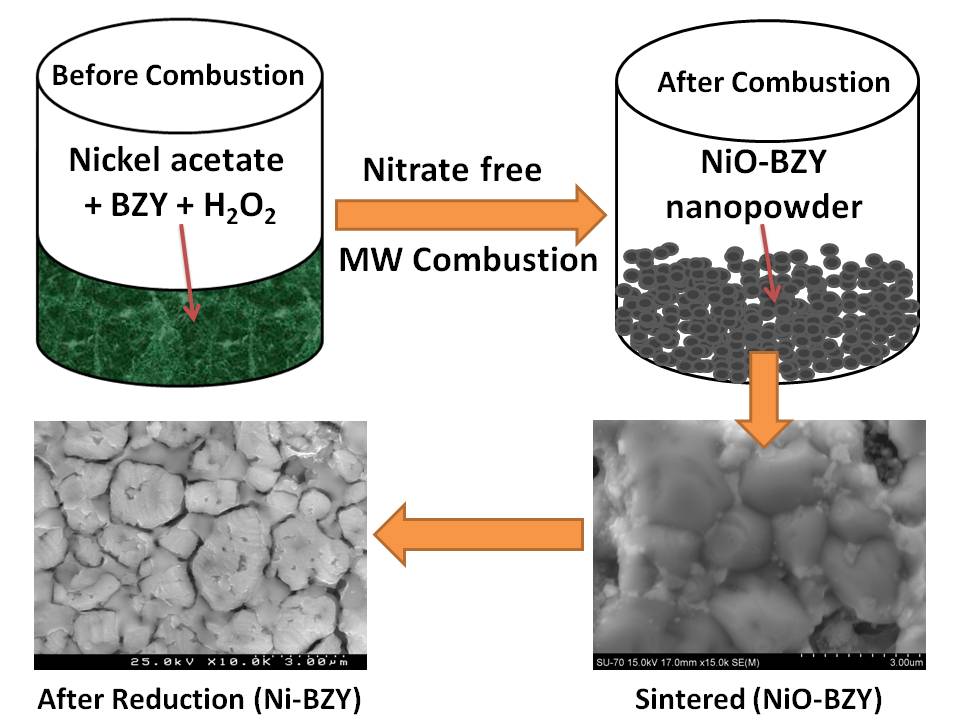
RSC Advances 2013, 3 (3), 859-869
Enhanced low temperature proton conduction in
Sr0.02La0.98NbO4-δ by scheelite phase retention.
A.D.Brandao, I.Antunes, J. R.Frade, J.Torre, V.V. Kharton, D.P.Fagg
The high-temperature scheelite phase of Sr0.02La0.98NbO4-δ materials has been retained to room temperature via vanadium substitution for the Sr0.02La0.98Nb1-xVxO4-δ compositional range of 0.25 = x = 0.325. Such structural stabilization avoids the characteristic break in the thermal expansion coefficient (TEC) that may otherwise be deleterious for the application of LaNbO4-based materials as proton-conducting electrolytes. The composition offers pure proton conduction with higher conductivity than the base composition in the low-temperature range under wet oxidizing conditions.
Chem. Mater., 2010, 22 (24), 6673–6683
Transport
Properties of Fluorite-Type Ce0.8Pr0.2O2-δ:
Optimization via the Use of Cobalt Oxide Sintering Aid
Duncan P. Fagg, Susana García-Martin, Vladislav V. Kharton, and Jorge R. Frade
Dense Ce0.8Pr0.2O2-δ
ceramic membranes with submicron grain size can be formed at 1000°C
by minor additions of cobalt oxide. The combination of enhanced
ambipolar conductivity and enhanced oxygen surface exchange kinetics
boosts oxygen permeability in 2 mol % cobalt oxide doped Ce0.8Pr0.2O2-δ
to one of the highest levels of oxygen permeability reported to date
for a single component mixed conducting fluorite material.

Chem. Mater., 2009, 21 (2), 381-391